Friday, December 29, 2006
Last post of 2006
A final top 5 of 2006:
Top 5 of discussed topics in Blogshire
1) J.J. La Clair (no explanation needed)
2) Chemblogging (no explanation needed)
3) Polonium 210 (no explanation needed)
4) Tenderbutton's death (see comments on 1, 2 and 3)
5) cooking (see for instance 'In the pipeline' and 'Dylan's collumn'; too lazy to add links)
I wish all my readers of this blog (all three of you) a great 2007 with beautiful crystals. good IC50 and nice physchem props.
Thursday, December 28, 2006
Alcohol abuse
 Right.. They mean you will become a smelly and mentally unstable begger when you drink too much.
Right.. They mean you will become a smelly and mentally unstable begger when you drink too much.They give a definition of alcohol abuse.
Friday, December 22, 2006
Christmas cactus
Nice to have this in a Bioorg. Med. Chem. article in December.

Tuesday, December 19, 2006
Article Titles Analysis
How good was the chemistry in 2006?
The greatest thing is that the efficiency of the chemistry is increasing every year.
 Efficient chemistry was done almost 300 times in 2006. It almost doubled in 6 years!
Efficient chemistry was done almost 300 times in 2006. It almost doubled in 6 years!Although the efficiency is increasing rapidly, it did not make the chemistry more convenient and not easier. The versatility has been quite steady.

If I were in management I would say that a target for 2007 is to try to translate the obtained efficiancy into more convenient and easy synthesis.
What was done exactly in 2006?
As we can see the best years for metathesis are over, and Suzuki-coupling managed to recover from the 2003 Suzuki-depression and is now taking a lead.
 In what medium is the chemistry done?
In what medium is the chemistry done?
 Apparently conventional solvents are losing terrain. The ionic-liquid mania is still going on, and aqueous and solvent-free reations are increasing rapidly as well. The green guys seem to be winning.
Apparently conventional solvents are losing terrain. The ionic-liquid mania is still going on, and aqueous and solvent-free reations are increasing rapidly as well. The green guys seem to be winning.
Conclusion: Efficient Suzuki-couplings were done in an environmental friendly manner but it did not make life easier.
Saturday, December 16, 2006
Moonshine
 This is not a picture of moonshine on the surface of juptier. It is my round-bottom flask.
This is not a picture of moonshine on the surface of juptier. It is my round-bottom flask. I had to clean my glassware because some amoebic sediment was having a party in my flask. The glassware had been in my shed for three weeks.
I had to clean my glassware because some amoebic sediment was having a party in my flask. The glassware had been in my shed for three weeks.I found other weird things there...
 No, it is not my haute cuisine insect soup. It is my oil bath.
No, it is not my haute cuisine insect soup. It is my oil bath.
Three weeks ago I distilled the last batch for this year. It takes some time before you can get drunk.
Starting with inexpensive and readily available sugar, the show begins.
You need : from left to right:
citric acid, yeast, yeast nutrient (diammonium phosphate), and sometimes activated coal.
 The sugar is cooked in water with citric acid to hydrolyse the glycosidic bond of sucrose turning it into invert sugar. The monosaccharides are fermented much better than the disaccharides. Oxygen rich water is added together with the nutrients and the yeast. The yeast starts to multiply. Ofcourse you use something like TurboYeast to obtain as much ethanol as possible (up to 18% can be reached using these types of yeast). The fermentation is done under anaerobic conditions.
The sugar is cooked in water with citric acid to hydrolyse the glycosidic bond of sucrose turning it into invert sugar. The monosaccharides are fermented much better than the disaccharides. Oxygen rich water is added together with the nutrients and the yeast. The yeast starts to multiply. Ofcourse you use something like TurboYeast to obtain as much ethanol as possible (up to 18% can be reached using these types of yeast). The fermentation is done under anaerobic conditions.
People say : "It is dangerous, watch out for the methanol. If you drink it, you will not be able to read the newspaper anymore!!" That is bullshit, methanol can not be formed when using pure sugar. It can be formed when fermenting fruit (pectins), but with a good still you can remove it with the the first fractions of the distillation.
With the TurboYeast it takes about two weeks to complete the fermentation. Now in December it is too cold here to do this. I usually ferment 10 litres every time. After fermentation you decantate the solution to remove the dead yeast and the first destillation is done. I have a three litre flask so I have to distill 4 times. (I do this in about 4 times 50 minutes). The 10 litres give about 1.7 litre of 70% ethanol after the first distillation. Sometimes you have a bit of nasty sulphur smell in it. This is often occuring when the yeast was too old, or the fermentation was left for too long. You can remove the nasty smell with activated coal (or by using a real copper still).
The purity of the ethanol is meassured with a density meter.
 Let it float in the distillate, and read the percentage.
Let it float in the distillate, and read the percentage. This one is only for ethanol water mixtures, so it will not work for wine wich has other ingredients. The water ethanol mixture can be diluted to about 40% to have a nasty shot of vodka.
This one is only for ethanol water mixtures, so it will not work for wine wich has other ingredients. The water ethanol mixture can be diluted to about 40% to have a nasty shot of vodka.I do not like vodka, so I flavour the ethanol with herbs. Crush the herbs in a mortar like an alchemist, and let it stand in the ethanol for a week.
I like (from left to right) : juniper berry, rosemary, tyme, nutmeg and pepper.
 After a week the herbs are filtered out and the ethanol is distilled again. I usually get 1.4 litres 80% flavoured ethanol. I dilute this to 40% to have 2.8 litres of nice jenever. Optionally you can mature the jenever on roasted chips of oak wood.
After a week the herbs are filtered out and the ethanol is distilled again. I usually get 1.4 litres 80% flavoured ethanol. I dilute this to 40% to have 2.8 litres of nice jenever. Optionally you can mature the jenever on roasted chips of oak wood. This will give the jenever a nice color and an extra flavour.
This will give the jenever a nice color and an extra flavour. The 2.8 litre of korenwijn-like spirit will cost you about $50 in a shop. The used ingredients for my 2.8 litre cost about $3 and an additional $5 for the butane/propane mixture to heat my oil bath. All the stuff can be bought easily. Biggest problem is the lab-glassware. You can make a still yourself from an old copper boiler, or milk can. Or search for real lab glassware on E-bay. You can not walk out of your lab with a 3 litre round bottom flask under your arm, I presume (you might want to keep your job).
The 2.8 litre of korenwijn-like spirit will cost you about $50 in a shop. The used ingredients for my 2.8 litre cost about $3 and an additional $5 for the butane/propane mixture to heat my oil bath. All the stuff can be bought easily. Biggest problem is the lab-glassware. You can make a still yourself from an old copper boiler, or milk can. Or search for real lab glassware on E-bay. You can not walk out of your lab with a 3 litre round bottom flask under your arm, I presume (you might want to keep your job).I have 9 bottles of this stuff right now, so I hope I can get through the winter. In april the show can begin again
Tuesday, December 12, 2006
Top 5 scandals in medicine and chemistry
Using the antisense method with phosphate-methylated DNA, Buck and Goudsmit claimed to have found a cure for AIDS and published their findings in a Science article. They held a press conference for the media to present their findings. The massive media attention made the scandal only worse. Colleagues discovered errors in their research, and it turned out that the phosphate-methylated DNA was not a stable compound, and the used samples could not have been pure.
It is said that Buck was a stubborn man who did not tolerate any resistance. His university did not immediately investigate the allegations of Buck’s colleagues, so the colleagues decided to go to the media with the supposed fraud in order to diminish the public optimism in the fight against AIDS. After this media attention the whole case was investigated. Buck was fired and Goudsmit rebuked.
2) Elias A. K. Alsabti
An Iraqi cancer specialist who published about 60 plagiarized papers in obscure journals with probably non-existing colleagues in order to boost his CV. While working in the USA it became clear that he had no real scientific knowledge. Eventually it seemed to be that his whole career was falsified.
3) Vijay Soman/Philip Felig
As peer reviewers for ‘The New England Journal of Medicine’ they rejected an article by Helena Wachslicht-Rodbard. They copied the paper with their own names on it and submitted it for publication in ‘The American Journal of Medicine’. Unfortunately for them, Helena Wachslicht-Rodbard was asked to peer review her own (stolen) article.
4) Jacques Benveniste
Benveniste published a paper in Nature that a homeopathically diluted solution of antibodies could activate white blood cells by the so-called ‘memory of water’. Benveniste failed in reproducing the results when John Maddox and James Randi investigated the work in Benveniste's lab.
Benveniste also claimed that the information that is stored by the ‘memory water’ could be transmitted over the internet or telephone lines. Benveniste was awarded the IgNobel prize twice.
5) Guido Zadel
He published a paper 'Enantioselective Reactions in a Static Magnetic Field‘ in Angewandte Chemie in 1994. It turned out that his results were falsified. Even results of his colleagues were falsified by replacement of samples. The results were part of his dissertation, and his PhD was eventually withdrawn.
Elena Ceauşescu
I didn’t read the book (polymer chemistry is no expertise of mine), but I saw the book came from Romania, and was indeed a book by THE Elena Ceauşescu. I rembered seeing her execution on TV a few years before. Inside the books of the library were lists of all people who borrowed it. This list was empty.
Fascinated by this historic document I searched the catalog and found out that almost all European university libraries had a copy of the book. It turned out that Ceauşescu had sent the book herself to the libraries.
Elena Ceauşescu was seeking for status, and with her husband’s influence she was able to obtain it. Her PhD in chemistry is probably false. It is disputed that she wrote the book herself, and it is said that she didn’t have the intellectual capabilities to obtain a PhD.
Scifinder gives 90 hits on her name. Starting with a patent, filed in 1964 with her name on the second place.

In 1965 the Romanian communist leader Gheorghe Gheorghiu-Dej died, and Nicolae Ceauşescu came to power. After that point, all other 89 patents and publications (Materiale Plastice/Revue Roumaine de Chimie) name Elena as the first author. Finally she had obtained a status as a scientist, and a status as the most hated person in Romania as well.
The last book she published (Dostizheniia v khimii i tekhnologii polimerov) appeared in 1988. A year later she probably sang : "Last Christmas".
Monday, December 11, 2006
Top 5 : Executed Chemists
Guillotined on May 8 1794 because of his job as a tax collector.
2) Elena Ceauşescu
Shot with a sub-machine-gun on December 25 1989, after the fall of her husband’s dictatorship in Romania.
3) Masami Tsuchiya
Sentenced to death on Januari 30 2004 because of his role in the Sarin gas attack on the Tokyo subway by Aum Shinrikyo.
4) Bruno Tesch
Executed on 16 May 1946 because of the production of Zyklon B for 'The Third Reich'.
5) Ernst Cohen
Executed (murdered) March 5 1944 in the gas chamber of Auschwitz.
Thursday, December 7, 2006
Evolution versus Creation
The Polish scientific community is concerned that this will effect biology teaching in schools through LPR-leader and Polish minister of education Roman Giertych and his father and LPR member of the European Parlaiment Maciej Giertych, the latter holds a PhD in tree physiology.
The article states that “neither Roman nor Maciej responded to Nature’s request for comment.”
Maciej Giertych however did comment in a later issue with the usual evolution criticism. Maciej Giertych’s correspondence received lot of criticism from the scientific community. His arguments are called pseudoscientific, and other arguments are said to be untrue. Several people state that Nature should not have published Maciej Giertych’s correspondence.
It is a difficult problem I think. On one hand Giertych should be able to comment on an article that mentions him, even if his comment contains scientific errors. On the other hand : Should Nature provide Giertych an audience to tell his pseudoscientific views that have political implications as well?
I don not know…
Pseudoscience can evolve into science. Alchemy became chemistry, although I can not see where this anti-evolution must lead to.
The Giertych’s contemptuous homophobic and aniti-Semitic opinion makes me think that you should ignore those guys. Unfortunately the Giertych-clan can not be ignored because of the positions they have.
My conclusion is that I hate it when science, religion and politics are mingling. It goes well when they live together. You can prove that the earth is not the centre of the universe while being a devout Christian. It goes wrong when the church and state interfere in your discovery, then you are silenced and can only mumble :”Eppur si muove”.
I think that both evolution and creation have to be taught in school, which of the theories is supported is up to the pupil. Both theories are important because of their value. Whether is a cultural, historic, religious or scientific value is not important for curriculum inclusion. Politics should not determine what is believed to be true.
Monday, December 4, 2006
Stupid things to do as a (Med.) Chemist
- Be a sales representative for Pfizer.
- Publish your optimized Suzuki reaction in water and tell the chemistry is oh so green, and hide the experimental procedure with the 5 litres dichloromethane extraction as a note between the references.
- Try to explain the biologist why the unreliable 6 step synthesis of the compound with 5 chiral centres takes much more time than the straightforward 8 step synthesis of another symmetrical compound.
- Explain your peasant neighbor what you do for a living.
- Criticize the senior management.
- Be honest about your trust in the validation of your target.
- After finishing a successful project, tell the boss that a project was much easier than expected.
Saturday, November 25, 2006
Spinning side band spectrometry
We had a problem with our NMR this week. The magnetic field homogeneity got worse within a few days resulting in the same spectrum for all our compounds : 9-0 ppm, multiplet, all H's. One TMS peak became three, and after a week we couldn't count the number of TMS peaks anymore.
 We are so lucky it got so bad. The manufacturer came yesterday and fixed the problem after a day kicking the magnets and a few hours of shimming.
We are so lucky it got so bad. The manufacturer came yesterday and fixed the problem after a day kicking the magnets and a few hours of shimming.
Wednesday, November 22, 2006
Top 5 chemistry relics
3.
"Photo 51" Sodium deoxyribose nucleate from calf thymus, Structure B, May 2, 1952
Mercury gasometer, used to identify chemical properties of oxygen, 1774

Original botlle of mauve dye, 1856
 Science Museum London, UK
Science Museum London, UKThis sample is most likely not the real first sample; "it cannot be earlier than 1862"
Tuesday, November 21, 2006
For the kids.
 Very nice for your kids to have in bed. Sweet dreams.
Very nice for your kids to have in bed. Sweet dreams.Monday, November 20, 2006
unnecessary idiom
Saturday, November 18, 2006
Some aqueous solution
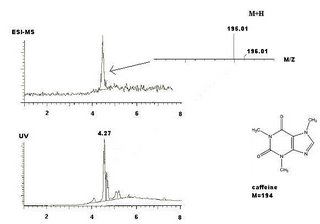
It turned out that caffeine gave a dominant peak in the chromatogram, and was the only peak with a clear MS-spectrum.
It will not surprise me if our boss just wants to make sure we work as fast as possible.

Friday, November 17, 2006
Kill your project ASAP
"These five companies, according to the report "deliver as much as a 17-month speed advantage over average performers"."
The reason is clear why other are so slow.
"Decisions still tend to be made at the highest possible level, which means decision-makers have less insight into what people are working on. Project teams go to the decision-makers fighting for their compounds as much as possible, and are reluctant to give up on their project, in the hope that a project will succeed through perseverance. This makes it easy for projects to run beyond the point where the team knows they are not going to work."
Wow.. What a conclusion… We all knew that already. This is how it works in all industries, and in politics as well.
Thursday, November 16, 2006
Who is who in chemicals and labware

Georg Büchner / Wilhelm Schlenk / Emil Erlenmeyer / Sir James Dewar / Ludwig Rainer Claisen
Wednesday, November 15, 2006
Mr. Vigreux
 Browsing through the book I see nice equipent I would like to have in my shed to prepare my own mooshine.
Browsing through the book I see nice equipent I would like to have in my shed to prepare my own mooshine.
Some illustration represent glassware that makes me wonder what it is used for. Unforntunately my French is far from perfect, and my fluently French speaking wife is not prepared to translate the 280 pages of instrument descriptions.
 Looking at all the glassware in this book I realize how spoiled we are in industry. Specific glassware becomes a rarity on our labs. The last vigreux I touched was last year, cracking dicyclopentadiene wich was the last destillation I did as well. You have to search the whole building for a complete destillation-set on the right scale. There is some mini-scale glassware that becomes mouldy and almost starts to decompose We are so spoiled we buy dry solvents, stored under nitrogen on molsieves. Ground-glass jointless glassware can nott be found here. Reagents like diazomethane are almost prohibited, we need 523 signatures and fill in 735 forms to be allowed to use such reagents. Apart from LDA we buy everything that can be made easily, because we have the presumptuous idea that our time is more expensive than the commercial reagents. It is a miracle we are able to synthesize anything at all.
Looking at all the glassware in this book I realize how spoiled we are in industry. Specific glassware becomes a rarity on our labs. The last vigreux I touched was last year, cracking dicyclopentadiene wich was the last destillation I did as well. You have to search the whole building for a complete destillation-set on the right scale. There is some mini-scale glassware that becomes mouldy and almost starts to decompose We are so spoiled we buy dry solvents, stored under nitrogen on molsieves. Ground-glass jointless glassware can nott be found here. Reagents like diazomethane are almost prohibited, we need 523 signatures and fill in 735 forms to be allowed to use such reagents. Apart from LDA we buy everything that can be made easily, because we have the presumptuous idea that our time is more expensive than the commercial reagents. It is a miracle we are able to synthesize anything at all.








